Prices for Abbott’s Norvir (generic name Ritonavir) as a Standalone Product in 2010
KEI Research Note 2010:4
August 12, 2010
Anne Mira Guha
Prices for Abbott’s Norvir (generic name Ritonavir) as a Standalone Product in 2010
1. Introduction
Ritonavir is a protease inhibitor (PI) used to treat the HIV infection. In a number of countries, ritonavir is patented and exclusively marketed by Abbott Labs under the trade name Norvir. While ritonavir was originally introduced as a “third drug” in a triple anti-retroviral (ARV) therapy regime for HIV/AIDS, it is now used to as an agent to enhance the effectiveness of other protease inhibitors. It is available in 100mg tablets, 100mg capsules, and as an oral solution. Most commonly prescribed are the pill forms (caps/tabs). The tablets, a relatively new presentation, are heat-resistant. The soft-gel capsules, an older presentation but still the most commonly available, require refrigeration. The oral solution, which comes in peppermint and caramel flavored vehicles, is often used for pediatric treatment.
For adults, the most common use is in doses of 100 to 200 mg per day, either as the standalone product Norvir, or co-formulated with lopinavir, a protease inhibitor combination treatment marketed by Abbott under the trade name Kaletra. In late 2003, Abbott “re-priced” Norvir in the United States, introducing a 400 percent increase when it is used as a standalone product. The price increase did not apply to countries outside the US, nor to the Abbott combination product Kaletra.
2. Recent Prices in High Income Countries
The following are prices from higher income countries where the ritonavir patent is in effect. (Prices are effective August 2010, unless otherwise noted.)
Table 1: Prices for 100 mg tablet or soft-gel capsule in high income countries
| Country |
Prices, August 2010 |
Price in USD [1]
(per pill) |
Annual Cost in USD
(at 2 pills a day) |
United States;
Average Retail Price |
$320.95 per 30 (same for caps & tabs) [2] |
$10.70 |
$7,811.00 |
| Denmark |
60,15 eur per 30 (caps) [3] |
$2.63 |
$1,919.90 |
| Norway |
384,60 kr per 30 (tabs) [4]
(includes VAT) |
$2.15 |
$1,569.50 |
| Austria |
Gross Retail Price: 133.04 euro for 84 (caps) [5] |
$2.04 |
$1,489.20 |
| Norway |
1014 kr per 84 (caps)
(includes VAT) |
$1.96 |
$1,430.80 |
| Sweden |
SEK 1063 per 84 (caps) [6] |
$1.80 |
$1,314.00 |
| Italy |
104.91 eur per 84 (caps) [7] |
$1.65 |
$1,204.50 |
| France |
PPTTC: 37,48 Euro per 30 (film-coated tablets) [8]
(PPTTC: price for the public with all taxes incl.) |
$1.61 |
$1,175.30 |
| Canada (Ontario) |
1.4671 CAD each (caps) [9] |
$1.42 |
$1,036.60 |
| Australia |
965.09 AUD per 720 (caps) [10] |
$1.42 |
$1,036.60 |
| Netherlands |
€ 1,07 each (same for tabs & caps) [11] |
$1.41 |
$1,029.30 |
| Australia |
103.82 AUD per 672 (tabs) |
$1.29 |
$941.70 |
| United States; Federal Supply Schedule [12] |
$35.90 per 30 (same for caps & tabs) |
$1.20 |
$876.00 |
| New Zealand |
$121.27 per 84 (caps) [13] |
$1.04 |
$759.20 |
The following are prices for the oral solution of Norvir in high income countries.
Table 2: Prices for oral solution in high income countries
| Country |
Exchg Rt |
Domestic Price |
ml |
mg/ml |
Price in USD for 100mg |
United States;
Average Retail Price |
— |
1,797,37 USD |
240 |
80 |
$9.36 |
| Italy |
(1.28) |
449.58 Euro |
450 |
80 |
$1.60 |
| Sweden |
(.135432) |
4110 SEK |
450 |
80 |
$1.55 |
Canada
(Ontario) |
(.954472) |
1.1839 CAD |
1 |
80 |
$1.41 |
| Australia |
(0.89489) |
986.12 AUD |
900 |
80 |
$1.41 |
| United States; Federal Supply Schedule |
— |
196.49 USD |
240 |
80 |
$1.02 |
3. Prices in Developing Countries
The following are prices for Norvir in developing countries taken from the MSF periodic surveys of ARV prices.
Table 3: Selected prices of tabs, capsules and oral solution in developing countries [14]
| Manuf. |
Presentation |
$/100mg
(i.e. 1 pill) |
Annual Cost
(200mg/day) |
Abbott |
RTV 100mg
soft-gel capsule |
$0.114 |
$83 |
| Abbott |
RTV 100mg
heat-stable capsule |
$0.114 |
$83 |
Abbott |
RTV 80mg/ml
oral solution |
$0.116
(0.093/ml) |
$85 |
| Cipla |
RTV 100mg
soft-gel capsule |
$0.442 |
$323 |
| Strides |
RTV 100mg
soft-gel capsule |
$0.300 |
$219 |
4. Comparison of U.S. to Foreign Prices
As demonstrated above, in August 2010, prices for Norvir were four to ten times higher in the United States than in other high income countries. The following graph shows the comparative annual costs of Norvir, calculated for a dose of two 100mg soft-gel capsules per day.
|
Figure 1, August 2010 annual cost of twice daily dose of 100 mg soft-gel capsules (in high income countries)
|
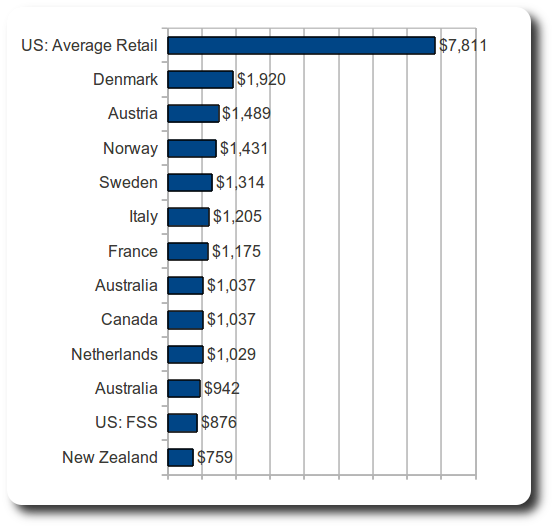
|
Recall from above that in developing countries a 100 mg dose of ritonavir is available for approximately $.11 to $.40 to national AIDS programs, resulting in an annual treatment price of $83-323.
5. U.S. Government Support for Development of Ritonavir
Ritonavir was invented by Abbott with Government support under contract number AI27220 awarded by the National Institute of Allergy and Infectious Diseases (NIAID).
An August 11, 2010 search of the NIH RePORT database using the search term “ritonavir” identifed 1,230 NIH grants. The RePORT database identifies 58 patents that have cited support from these grants.
An August 11, 2010 search of the USPTO database using the search terms “ritonavir” and “government” identifies 370 patents.
An August 11, 2010 search of ClinicalTrials.Gov identifies 130 trials that were funded by the NIH or other U.S. federal agencies. 181 trials were identified as funded by a university or other non-profit entity. 289 trials were identified as having received funding from industry. These results include 77 cases where trials were jointly funded. The total number of trials identified was 523.
Following the 400 percent price increase in the United States, in 2004, Essential Inventions asked the NIH to grant an open license for use of ritonavir in the United States, in a case concerning the March-In rights of the Bayh-Dole Act. The NIH rejected this request in August 2004.
6. Notes
[1] currency conversions done at http://www.xe.com/ucc
[2] http://www.drugstore.com/pharmacy/drugindex/rxsearch.asp?search=norvir
[3] http://www.medicinpriser.dk/Default.aspx?id=15&vnr=036290
[4] http://www.felleskatalogen.no/
[5] http://cedd.oep.hu/
[6] http://www.fass.se/LIF/produktfakta/artikel_produkt.jsp?NplID=19960826000029&DocTypeID=30
[7] http://farmaco.agenziafarmaco.it/index.php
[8] http://www.legifrance.gouv.fr/affichTexte.do?cidTexte=JORFTEXT000022389700
[9] https://www.healthinfo.moh.gov.on.ca/formulary/SearchServlet
[10] http://www.pbs.gov.au/html/home
[11] http://www.medicijnkosten.nl/
[12] See http://www.pbm.va.gov/DrugPharmaceuticalPrices.aspx, which provides the following explanation:
The FSS (Federal Supply Schedule) is a multiple award, multi-year federal contract that is available for use by any Federal Government agency. It satisfies all Federal contract laws and regulations. Pricing is negotiated based on how vendors do business with their commercial customers.
The FSS program also provides additional opportunities for savings to the customers with negotiated quantity and tier discounts.
For those products covered under Public Law 102-585, Veterans Health Care Act of 1992, pricing is either negotiated based on vendor’s most favored commercial customer pricing or statutorily-required pricing calculations. Vendors have an opportunity to establish FSS Big 4 prices and FSS dual prices. Big 4 prices are only available to VA, Department of Defense, Public Health Service (Indian Health Service), and U.S. Coast Guard customers and are based on pricing calculations outlined under the Public Law. Dual prices are negotiated for Other Government Agencies (OGAs) that comprise the remaining authorized users of the FSS program. Dual prices are based on most favored commercial customer pricing negotiations held with the vendors.
[13] http://www.pharmac.govt.nz/Schedule?q=norvir
[14] Untangling the Web of Antiretroviral Price Reductions: 13th (July 2010) and 12th editions (January 2010); http://www.msfaccess.org/resources/key-publications/
Response by Abbott
Abbott was given an opportunity to review and comment on this research note. On September 3, 2010, Abbott indicated it would not provide a comment on the report, via an email from Elizabeth Hoff, Senior Manager of Pharmaceutical and Public Affairs for Abbott.
