Public and Private Sector Funded Research for Fabry’s Disease
KEI Research Note 2010:2
23 July 2010
Introduction
Fabry’s disease is a rare genetic multisystemic disorder associated with progressive lipid accumulation and cell damage, leading to a variety of aliments, including some that are life-threatening, including kidney failure, heart attacks and strokes. The disease was identified in 1898 independently by both Dr William Anderson in England and Dr Johannes Fabry in Germany. Other names for the disease include:
- Alpha-galactosidase A deficiency
- Anderson-Fabry disease
- Diffuse angiokeratoma
One important but extremely expensive treatment for Fabry’s disease is Fabrazyme (agalsidase beta) enzyme replacement therapy. The FDA approved Fabrazyme for Fabry’s disease in 2003. The development of Fabrazyme was supported by U.S. government funding through the NIH. Fabrazyme is manufactured and marketed by Genyzme, under a patent license from The Mount Sinai School of Medicine.
The cost of Fabrazyme varies according to patient weight. For some patients, it will cost approximately $270,000 per year, or more than $700 per day.
In December 2009, another firm, Shire, filed a BLA for Replagal, another expensive biologic drug that will compete directly with Fabrazyme. From a December 2009 Shire press release:
REPLAGAL is currently approved for the treatment of Fabry disease in 45 countries and has been available to U.S. patients since December 2009 under an FDA-approved treatment protocol filed at the request of FDA. The REPLAGAL early access program was put in place as a result of the supply disruption of the only currently marketed treatment for Fabry disease in the U.S.
For earlier reports on the competition for Fabry’s disease enzyme replacement therapy, see reports here.
At present, Genyzme is facing problems manufacturing Fabrazyme, resulting in shortages of the medicine.
This research note will examine the role of the federal government in the development of Fabrazyme and Replagl, as well as other treatments for Fabry’s disease.
The NIH Research Portfolio Online Reporting Tools (RePORT) database
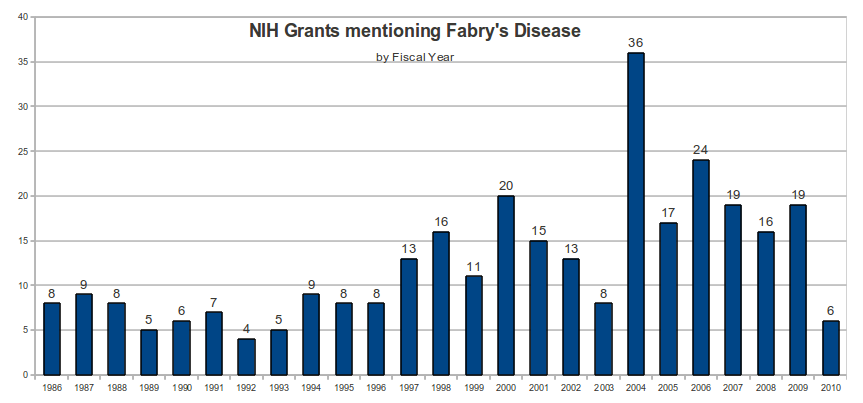
On July 22, 2010, a search of the NIH Research Portfolio Online Reporting Tools (RePORT) database using the keyword “Fabry” identified 372 NIH grants.
Genyzme reports on its 2009 SEC 10-K report that:
Fabrazyme is protected by U.S. Patent No. 5,356,804, which is licensed from Mount Sinai School of Medicine of the City of New York and expires on September 27, 2015.
Associated with the 372 NIH grants are 29 reported patents (See Appendix), of which 7 patents included a specific reference to Fabry’s disease.
The Inventors and Assignees and NIH Bayh-Dole Rights for the 7 patents are as follows:
- United States Patent 5,356,804
October 18, 1994
Cloning and expression of biologically active human .alpha.-galactosidase A
Inventors: Desnick; Robert J. (New York, NY), Bishop; David F. (New York, NY), Ioannou; Yiannis A. (New York, NY)
Assignee: Mount Sinai School of Medicine of the City of New York (New York, NY)
Filed: October 24, 1990
Government rights:This invention was made with government support under grant No. DK-34045 awarded by the National Institutes of Health. The Government has certain rights in the invention.
This patent was assigned to the NIH on September 13, 1991. - United States Patent Number 5,382,524
January 17, 1995
Cloning and expression of biologically active .alpha.-n-acetylgalactosaminidase
Inventors: Desnick; Robert J. (New York, NY), Bishop; David F. (New York, NY), Ioannou; Yiannis A. (New York, NY), Wang; Anne M. (New York, NY)
Assignee: The Mount Sinai School of Medicine of the City University of New York (New York, NY)
Filed: October 24, 1990
Government rights:Despite identification by the NIH Report database, none declared in the patent legend.
However, the patent was assigned to the NIH on April 7, 1995. - United States Patent Number 5,401,650
March 28, 1995
Cloning and expression of biologically active .alpha.-galactosidase A
Inventors: Desnick; Robert J. (New York, NY), Bishop; David F. (New York, NY), Ioannou; Yiannis A. (New York, NY)
Assignee: The Mount Sinai School of Medicine of the City University of New York (New York, NY)
Filed: November 30, 1992
Government rights:Despite identification by the NIH Report database, none declared in the patent legend.
However, the patent was assigned to the NIH on January 25, 1995. - United States Patent Number 5,491,075
February 13, 1996
Cloning and expression of biologically active.alpha.-N-acetylgalactosaminidase
Inventors: Desnick; Robert J. (New York, NY), Bishop; David F. (New York, NY), Ioannou; Yiannis A. (New York, NY), Wang; Anne M. (New York, NY)
Assignee: The Mount Sinai School of Medicine of the City University of New York (New York, NY)
Filed: June 17, 1994
Government rights:Despite identification by the NIH Report database, none declared in the patent legend.
However, the patent was assigned to the NIH on January 25, 1995. - United States Patent Number 5,580,757
December 3, 1996
Cloning and expression of biologically active .alpha.-galactosidase A as a fusion protein
Inventors: Desnick; Robert J. (New York, NY), Bishop; David F. (New York, NY), Ioannou; Yiannis A. (New York, NY)
Assignee: The Mount Sinai School of Medicine of the City University of New York (New York, NY)
Filed: June 17, 1994
Government rights:Despite identification by the NIH Report database, none declared in the patent legend.
However, the patent was assigned to the NIH on January 25, 1995. - United States Patent Number 6,455,037
September 24, 2002
Cells expressing an .alpha.gala nucleic acid and methods of xenotransplantation
Inventors: Ioannou; Yiannis (New York, NY), Desnick; Robert J. (New York, NY), Sandrin; Mauro S. (Brunswick, AU), McKenzie; Ian F. C. (Brunswick, AU)
Assignee: Mount Sinai School of Medicine of the City University of New York (New York, NY)
The Austin Research Institute (Heidelberg, AU)
Filed: November 1, 1996
Government Rights:This work was supported in part by an NIH grant (No. DK3045).
This patent was assigned to the NIH on June 3, 1997. - United States Patent Number 7,148,251
December 12, 2006
Amino ceramide-like compounds and therapeutic methods of use
Inventors: Shayman; James A. (Ann Arbor, MI)
Assignee: The Regents of the University of Michigan (Ann Arbor, MI)
Filed: January 10, 2002
Government rights:SPONSORSHIP : Work on this invention was sponsored in part by National Institutes of Health Grant R01 DK55823. The Government may have certain rights in the invention.
This patent was assigned to the NIH on March 23, 2005.
Data from ClinicalTrials.Gov
A July 23, 2010 search of clinicaltrials.gov using the key words “Fabry’s Disease” identified 54 clinical trials.
- 14 trials received funding from the NIH.
- 16 trials received funding from Universities or other non-profit organizations.
- 27 trials received funding from industry.
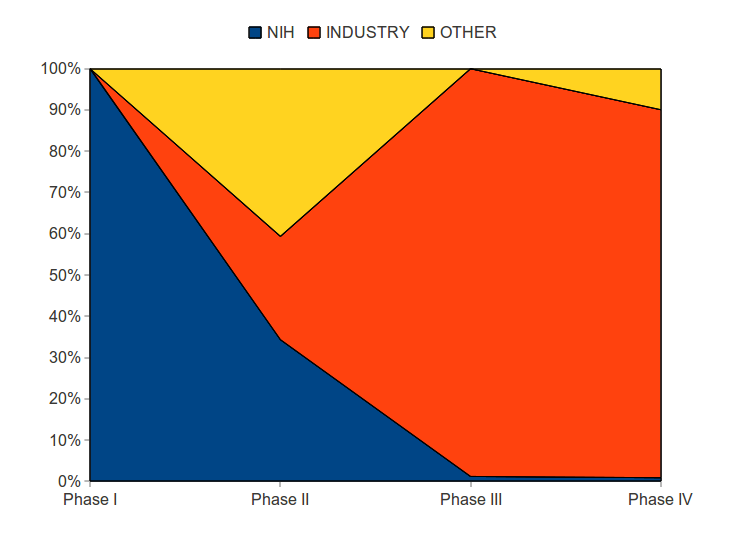
Particularly important for drug development are the Phase I, II, III trials.
Number of Phase I-III Trials, by funder
| Phases | NIH | Industry* | Other** | Total |
| Phase I | 1 | 1 | ||
| Phase II | 5 | 9 | 2 | 16 |
| Phase III | 1 | 5 | 6 | |
| Total Phase I-III: | 7 | 14 | 2 | 23 |
Enrollment in Phase I-III Trials, by funder
| Phases | NIH | Industry* | Other** | Total |
| Phase I | 120 | 120 | ||
| Phase II | 196 | 143 | 232 | 571 |
| Phase III | 3 | 249 | 252 | |
| Total Phase I-III | 319 | 392 | 232 | 942 |
Phase IV trials are done after a product enters the market, and can be important in evaluating the ongoing evidence of safety and efficacy of products.
| NIH | Industry* | Other** | Total | |
| Number of Phase IV Trials | 1 | 7 | 2 | 10 |
| Enrollment in Phase IV trials | 25 | 2546 | 284 | 2855 |
* Includes Industry/Unknown
* Includes Network/Other
In general, the NIH paid for the most risky phases of drug development. The industry share of drug development costs was small in the beginning, but grew as the commercial viability of the product was established.
The NIH paid for all Phase I trials and 34 percent of Phase II trials. Industry paid for none of Phase 1 trials, 25 percent of Phase II trials, and 99 percent of Phase III trials. The industry also dominated funding for Phase IV — trials often associated with marketing efforts.
Trials Identified on the FDA Label for Fabrazyme
The FDA label for Fabrazyme makes reference to four clinical trials to study the drug’s safety and efficacy, including one Phase II, one Phase III trial, and two Phase IV trials. All four were sponsored and funded by Genzyme.
- Study 1 was a randomized, double-blind, placebo-controlled, multi-national, multi-center study of 58 Fabry patients (56 males and 2 females), ages 16 to 61 years, all naïve to enzyme replacement therapy. The trial is reported in ClinicalTrials.Gov as: NCT00074971.
A Study of the Safety and Efficacy of Fabrazyme in Patients With Fabry Disease
Condition: Fabry Disease
Intervention: Drug: Fabrazyme (agalsidase beta)
Sponsor: Genzyme
Phase: Phase III
Number Enrolled: 58
Funded By: INDUSTRY
Study Design: Allocation: Non-Randomized; Control: Uncontrolled; Endpoint Classification: Safety/Efficacy Study; Intervention Model: Single Group Assignment; Masking: Open Label; Primary Purpose: Treatment
NCT ID: NCT00074971
First Received Date: December 24, 2003
Start Date: October 1999
Completion Date: December 2004 - Study 2 was a randomized (2:1 Fabrazyme to placebo), double-blind, placebo-controlled, multi-national, and multicenter study of 82 patients (72 males and 10 females), ages 20 to 72 years, all naïve to enzyme replacement therapy. Sixty-seven patients who participated in Study 2 were subsequently entered into an open-label extension study in which all patients received 1 mg/kg of Fabrazyme every two weeks for up to a maximum of 18 months.
- Study 3 (Pediatric Study) was an open-label, uncontrolled, multi-national, multi-center study to evaluate safety, pharmacokinetics, and pharmacodynamics of Fabrazyme treatment in 16 pediatric patients with Fabry disease (14 males, 2 females), who were ages 8 to 16 years at first treatment.
Study 3 is identified in ClincialTrials.Gov as: NCT00074958
Condition: Fabry Disease
Intervention: Biological: Fabrazyme (agalsidase beta)
Sponsor: Genzyme
Phase: Phase II
Number Enrolled: 16
Funded By: INDUSTRY
Study Design: Allocation: Non-Randomized; Control: Uncontrolled; Endpoint Classification: Safety Study; Intervention Model: Single Group Assignment; Masking: Open Label; Primary Purpose: Treatment
NCT ID: NCT00074958
First Received Date: December 24, 2003
Start Date: October 2002
Completion Date: July 2005
Primary Completion Date: May 2005 - Study 4 was an open-label, re-challenge study to evaluate the safety of Fabrazyme treatment in patients who had a positive skin test to Fabrazyme or who had tested positive for Fabrazyme-specific IgE antibodies. In this study, six adult male patients, who had experienced multiple or recurrent infusion reactions during previous clinical trials with Fabrazyme, were re-challenged with Fabrazyme administered as a graded infusion, for up to 52 weeks of treatment.
Study 4 was identified in ClinicalTrials.Gov as NCT00140621:
A Safety and Efficacy Study of Fabrazyme® Replacement Therapy in Patients With Cardiac Fabry Disease
Condition: Fabry Disease
Intervention: Drug: Agalsidase beta (Fabrazyme)
Sponsor: Genzyme
Phase: Phase IV
Number Enrolled: 6
Funded By: INDUSTRY
Study Design: Allocation: Non-Randomized; Control: Uncontrolled; Endpoint Classification: Safety/Efficacy Study; Intervention Model: Single Group Assignment; Masking: Open Label; Primary Purpose: Treatment
NCT ID: NCT00140621
First Received Date: August 30, 2005
Start Date: July 2005
Completion Date: June 2012
Primary Completion Date: June 2012
Study 2 was identified in ClincialTrials.Gov as: NCT00074984:
A Study of the Safety and Efficacy of Fabrazyme as Compared to Placebo in Patients With Advanced Fabry Disease
Condition: Fabry Disease
Interventions: Drug: Fabrazyme (agalsidase beta); Drug: Placebo
Sponsor: Genzyme
Phase: Phase IV
Number Enrolled: 82
Funded By: INDUSTRY
Study Design: Allocation: Randomized; Control: Placebo Control; Endpoint Classification: Safety/Efficacy Study; Intervention Model: Parallel Assignment; Masking: Double Blind (Subject, Caregiver, Investigator, Outcomes Assessor); Primary Purpose: Treatment
NCT ID: NCT00074984
First Received Date: December 24, 2003
Start Date: February 2001
Completion Date: June 2004
Primary Completion Date: January 2004
Like many orphan products, the number of patients in the trials was small, relative what would be required for many other products.
| Enrollment | |
| Phase II | 16 |
| Phase III | 58 |
| Phase IV | 88 |
For the critical Phase II-III trials, which were the basis of the FDA approval in 2003, the total number of patients was just 84. Compare this to the average of 5,303 patients in clinical trials for drug approvals reported in a widely cited 2003 article by Joseph DiMasi and others.
“we have found as the mean number of subjects across all three clinical phases for the investigational drugs in our cost survey (5303). Joseph A. DiMasi, Ronald W. Hansen and Henry G. Grabowski, “The price of innovation: new estimates of drug development costs,” Journal of Health Economics 22 (2003) 151–185.
DiMasi et al also cited a 2000 CMR report that found the mean number of subjects to be 4478 for 23 marketing approval applications submitted from 1995 to 2000.” (Describing Dossiers: Characterising Clinical Dossiers for Global Registration. R&D Briefing 25, CMR International, Surrey, UK.)
Orphan Drug Act Subsidies
Genzyme was able to receive a tax credit equal to half the cost of the clinical trials for Fabry’s Disease, under the U.S. Orphan Drug Tax Credit.
Appendix
Patents associated with NIH grants on Fabry’s disease*
*July 22, 2010 search of NIH RePORT
|
Core Project Number |
Patent Number |
Patent Title |
|
M01RR000037 |
7138389 |
Oral androgen therapy using modulators of testosterone bioavailability |
|
M01RR000039 |
6228839 |
Use of keratinocyte growth factor to improve oxidative status |
|
M01RR000188 |
7160676 |
Method of determining sperm capacitation |
|
M01RR000833 |
5106837 |
Adenosine derivatives with therapeutic activity |
|
M01RR000833 |
5234811 |
Assay for a new gaucher disease mutation |
|
M01RR000833 |
5271931 |
Methods for increasing C1 inhibitor concentrations using interferon-gamma and/or interleukin-6 |
|
M01RR000833 |
5424296 |
2-Halo-2′-deoxyadenosines as therapeutic agents against malignant astrocytoma |
|
M01RR000833 |
5506214 |
Use of substituted adenine derivatives for treating multiple sclerosis |
|
P41RR002594 |
5280788 |
Devices and methods for optical diagnosis of tissue |
|
P41RR002594 |
5312396 |
Pulsed laser system for the surgical removal of tissue |
|
P41RR002594 |
5419323 |
Method for laser induced fluorescence of tissue |
|
P41RR002594 |
5452723 |
Calibrated spectrographic imaging |
|
P41RR002594 |
5562100 |
Method for laser induced fluorescence of tissue |
|
P41RR002594 |
5919140 |
Optical imaging using time gated scattered light |
|
P41RR002594 |
6321111 |
Optical imaging using time gated scattered light |
|
P41RR002594 |
6404497 |
Polarized light scattering spectroscopy of tissue |
|
P41RR002594 |
6537211 |
Flourescence imaging endoscope |
|
P41RR002594 |
6611339 |
Phase dispersive tomography |
|
P41RR002594 |
6624890 |
Polarized light scattering spectroscopy of tissue |
|
P41RR002594 |
6690966 |
Methods of molecular spectroscopy to provide for the diagnosis of tissue |
|
P41RR002594 |
6697652 |
Fluorescence, reflectance and light scattering spectroscopy for measuring tissue |
|
P41RR002594 |
6697665 |
Systems and methods of molecular spectroscopy to provide for the diagnosis of tissue |
|
R01DK034045 |
5356804 |
Cloning and expression of biologically active human .alpha.-galactosidase A |
|
R01DK034045 |
5382524 |
Cloning and expression of biologically active .alpha.-n-acetylgalactosaminidase |
|
R01DK034045 |
5401650 |
Cloning and expression of biologically active .alpha.-galactosidase A |
|
R01DK034045 |
5491075 |
Cloning and expression of biologically active .alpha.-N-acetylgalactosaminidase |
|
R01DK034045 |
5580757 |
Cloning and expression of biologically active .alpha.-galactosidase A as a fusion protein |
|
R01DK034045 |
6455037 |
Cells expressing an .alpha.gala nucleic acid and methods of xenotransplantation |
|
R01DK055823 |
7148251 |
Amino ceramide-like compounds and therapeutic methods of use |