PDF version of letter to NIH. Failure2disclose-Doudna-patents-12oct2020
October 12, 2020
Michelle Bulls
Director
Office of Policy for Extramural
Research Administration (OPERA)
National Institutes of Health
Via Email: michelle.bulls@nih.gov
RE: Failures to disclose government funding associated with patents related to “Methods and compositions for RNA-directed target DNA modification and for RNA-directed modulation of transcription.”
Dear Ms. Bulls:
Knowledge Ecology International (KEI) requests that the National Institutes of Health (NIH) investigate the apparent failure to disclose federal support of several patents directed to methods and compositions for RNA-directed target DNA modification and for RNA-directed modulation of transcription, in violation of the Bayh-Dole Act, 35 U.S.C. §§ 200 et seq. The patents, all titled “Methods and compositions for RNA-directed target DNA modification and for RNA-directed modulation of transcription,”[fn 1] are available in the Annex I of this letter.
/fn 1/ One of the patents, U.S. patent 10,400,253, has the following title “Methods and compositions or RNA-directed target DNA modification and for RNA-directed modulation of transcription” [emphasis added], but we assume the “or” instead of a “for” is a typo, since the title would otherwise make little sense.
The inventors listed on each of the Annex I patents are Jennifer A. Doudna, Martin Jinek, Krzysztof Chylinski, and Emmanuelle Charpentier. Each of the Annex I patents are assigned to the Regents of the University of California, University of Vienna, and Emmanuelle Charpentier. These patents are part of Berkeley’s portfolio directed to CRISPR-Cas9. Recently, Dr. Doudna and Dr. Charpentier received the Nobel Prize in Chemistry for their CRISPR discoveries.
Evidence suggests that the inventions covered in the Annex I patents benefited from grant number GM081879, awarded by the National Institute of General Medical Sciences (NIGMS). Yet, none of them disclose government funding, as they are required to do under federal law.
When federal grants support a new technology, the grant recipient must make certain disclosures to the funding agency. These disclosures alert the government to the rights that it retains in federally-sponsored inventions, including a “nonexclusive, nontransferrable, irrevocable, paid-up license to practice or have practiced for or on behalf of the United States any subject invention throughout the world,” 35 U.S.C. § 202(c)(4), and the right to “march-in” and compel additional licensing of an invention when, among other reasons, the contractor, assignee or licensee has not taken steps to achieve practical application, 35 U.S.C. § 203(a).
Failure to comply with the Bayh-Dole Act’s reporting requirements undermines one of the stated policy objectives of the legislation: “to ensure that the Government obtains sufficient rights in federally supported inventions to meet the needs of the Government and protect the public against nonuse or unreasonable use of inventions.” 35 U.S.C. § 200. If an agency is not aware of inventions it has supported, it cannot ensure that the objectives of the Act are being met./fn 2/
/fn 2/ Department of Health and Human Services Office of Inspector General, Underreporting Federal Involvement in New Technologies Developed at the Scripps Research Institute (June 15, 1994).
We respectfully urge the NIH to investigate the apparent non-disclosures and take appropriate remedial action, including asserting the government’s rights in the patented inventions.
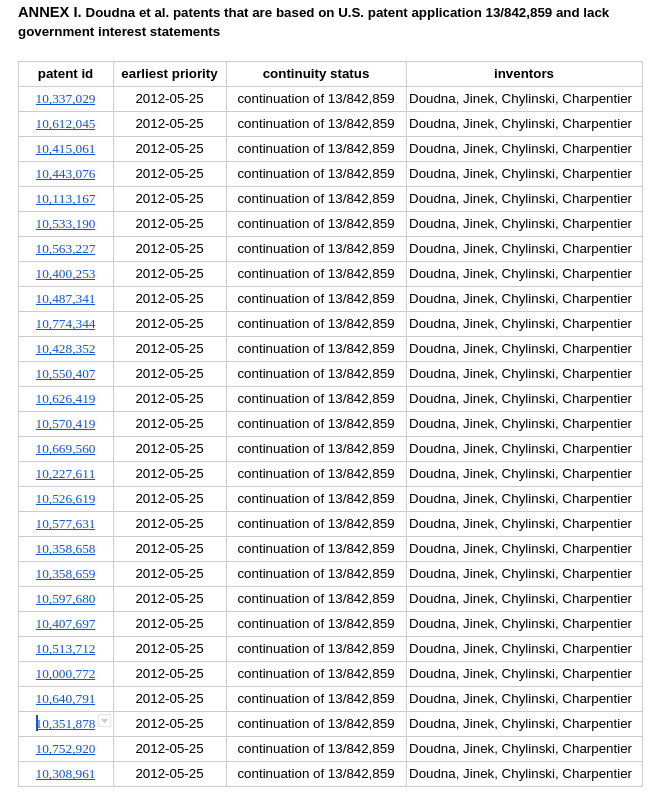
These patents are all members of the same family
As explained below, KEI has discovered evidence to suggest that the patents listed in Annex I fail to disclose government funding. Specifically, KEI has identified 33 U.S. patents that share the same priority numbers, inventors, and assignees; derive from the same original application, U.S. Patent Application 13/842,859; and are all linked to the same USPTO Patent Family ID. However, five of those patents have a government interest statement and the rest do not.
The Annex I patents are all continuations of U.S. Patent Application 13/842,859, filed March 15, 2013. These patents all claim the benefit of U.S. Provisional Patent Application Nos. 61/652,086; filed May 25, 2012; 61/716,256, filed October 19, 2012; 61/757,640, filed January 28, 2013; and 61/765,576, filed February 15, 2013. These patents all have the same Patent Full-Text and Image Database (PATFT) Patent Family ID number – 49624232./fn 3/
/fn 3/ The USPTO describes the Patent Family ID search field as follows: “The Patent Family ID correlates all members of the family under a unique Family ID. Members of the family include PG-Pubs, US-Patents, foreign references, continuational, and divisional applications and patents.” See: http://patft.uspto.gov/netahtml/PTO/help/helpflds.htm#FMID.
U.S. Patent Application 13/842,859, which served as the basis for the Annex I patents, as their own continuity data section explains, has the following government interest statement: “This invention was made with government support under Grant No. GM081879 awarded by the National Institutes of Health. The government has certain rights in the invention.”
Failure to disclose in the ‘167 and ‘772 patents
Two of the patents in the Annex I are U.S. patents 10,113,167 (the ‘167 patent) and 10,000,772 (the ‘772 patent). With regards to the ‘167 and ‘772 patents, the primary evidence of failure to disclose stems from the simple fact that they are currently listed in the NIH Project RePORTER database entry for grant number P50GM081879. If they were reported throughout the iEdison and are listed in RePORTER, they must also have government interest statements. All that the NIH needs to do to confirm this is to consult the RePORTER database for themselves.
Furthermore, at least one of these two documents, the ‘772 patent, has a confirmatory license to the NIH executed on March 15, 2017. /fn 4/ Confirmatory licenses are a requirement under Executive Order 9424. The fact that this patent has a registered confirmatory license further confirms that it was also subject to the government interest statement requirements, but failed to meet them.
/fn 4/ http://legacy-assignments.uspto.gov/assignments/assignment-pat-42038-919.pdf
Failure to disclose for the remaining Annex I patents
With regards to the remaining Annex I patents, the primary evidence of failure to disclose is the fact they are all continuations of U.S. patent application 13/842,859, filed March 15, 2013, and issued as U.S. patent 10,266,850. The 13/842,859 application contains the following statement:
“This invention was made with government support under Grant No. GM081879 awarded by the National Institutes of Health. The government has certain rights in the invention.”
A continuing patent application is “an application filed subsequently to another application, while the prior application is pending, disclosing all or a substantial part of the subject-matter of the prior application and containing claims to subject-matter common to both applications, both applications being filed by the same inventor or his legal representative.” U.S. Water Servs., Inc. v. Novozymes A/S, 843 F.3d 1345, 1348 (Fed. Cir. 2016). By definition, the subject matter in all of the Annex I patents is related to an application that disclosed government interest.
Bayh-Dole disclosure requirements
The Bayh-Dole Act and federal regulations and guidelines make clear several obligations for contractors in the disclosure of government rights in subject inventions, including: (1) a requirement to disclose that federal funding contributed to an invention; (2) NIH contractual requirements for disclosure; and (3) required language to be inserted in patent applications and patents, stating the role of federal funding and the government’s rights.
Under 35 U.S.C. § 202(c)(1), any contractor that receives funding from the federal government is required to “disclose each subject invention to the Federal agency within a reasonable time after it becomes known to contractor personnel responsible for the administration of patent matters.”
Under 37 C.F.R. § 401.3(a), each federal funding agreement shall contain the “standard patent rights clause” found at 37 C.F.R. § 401.14, barring specific circumstances and exceptions. Subsection (c)(1) of the patent rights clause outlines the disclosure requirements.
37 C.F.R. § 401.14(c)(1)
(c) Invention Disclosure, Election of Title and Filing of Patent Application by Contractor
(1) The contractor will disclose each subject invention to the Federal Agency within two months after the inventor discloses it in writing to contractor personnel responsible for patent matters. The disclosure to the agency shall be in the form of a written report and shall identify the contract under which the invention was made and the inventor(s). It shall be sufficiently complete in technical detail to convey a clear understanding to the extent known at the time of the disclosure, of the nature, purpose, operation, and the physical, chemical, biological or electrical characteristics of the invention. The disclosure shall also identify any publication, on sale or public use of the invention and whether a manuscript describing the invention has been submitted for publication and, if so, whether it has been accepted for publication at the time of disclosure. In addition, after disclosure to the agency, the Contractor will promptly notify the agency of the acceptance of any manuscript describing the invention for publication or of any on sale or public use planned by the contractor.
According to 37 C.F.R. § 401.14(f)(4) and NIH Guidelines for Grants and Contracts, grant recipients must include the following language in their patent applications and patents:
“This invention was made with Government support under (grant/contract number) awarded by the (Federal agency). The Government has certain rights in the invention.”
Finally, under 35 U.S.C. § 202(c)(6) and 37 C.F.R. § 1.77(b)(3), contractors are required to state within the patent application or patent that the federal government contributed funding to support the discovery of the invention and that the government retains certain rights.
35 U.S.C. § 202(c)(6)
(c) Each funding agreement with a small business firm or nonprofit organization shall contain appropriate provisions to effectuate the following:
(6) An obligation on the part of the contractor, in the event a United States patent application is filed by or on its behalf or by any assignee of the contractor, to include within the specification of such application and any patent issuing thereon, a statement specifying that the invention was made with Government support and that the Government has certain rights in the invention.
Remedies
KEI urges the NIH to promptly investigate the apparent non-disclosure and take all appropriate remedial action, including taking title to the patents. Failure to disclose subject inventions pursuant to 35 U.S.C. § 202(c)(1) permits the federal government to “receive title to any subject invention not disclosed to it within such time[.]”
If the NIH never revokes a patent or imposes any meaningful sanctions, universities and other contractors will continue to underreport federal funding. A certificate of correction on the patent does not send a strong enough signal regarding the public’s right to know its rights in patented inventions.
The disclosure requirements were designed to protect the government’s rights in federally-sponsored technology such as biomedical inventions. We are asking the NIH to ensure that when taxpayer investments are relevant to an invention, the government is diligent in ensuring that the public’s rights are acknowledged and secured. In order to protect the public’s rights, we ask that the government take possession of patents when grant recipients do not make timely disclosures, as is required by law, and as appears to be the case here.