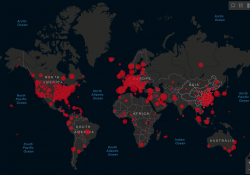
Open letter asking 37 WTO Members to declare themselves eligible to import medicines manufactured under compulsory license in another country, under 31bis of TRIPS Agreement
Background In 2001, the World Trade Organization (WTO) began negotiations on the rules regarding patents and access to medicine. While several issues were clarified and resolved in the November 2001 “Doha Declaration on TRIPS and Public Health”, the negotiations took… Continue Reading