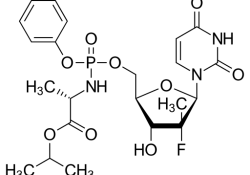
EC publishes report on protection and enforcement of intellectual property rights in “third countries”
On March 12, 2018, the European Commission issued a press release on a report on the “protection and enforcement of intellectual property rights in third countries”. The report was published on February 21, 2018 and is entitled – “Commission Staff… Continue Reading