Malaysia . March 14, 2018 Letter to USTR re Malaysia Special 301
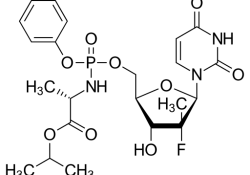
These were KEI comments to USTR regarding PhRMA’s efforts to target Malaysia as a Priority Foreign Country for issuing a compulsory license on sofosbuvir patents. Malaysia.14March2018.KEI.USTR.Special 301 March 14, 2018 The Honorable Robert Lighthizer United States Trade Representative Executive Office… Continue Reading