13 NGOs Call on USTR To Support Colombia in Special 301 Following Pressure Over Legal Access to Meds Efforts
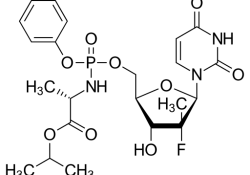
KEI submitted a post-hearing submission to the USTR for the Special 301 on behalf of itself and twelve other international NGOs and academic institutions condemning recent pressures on Colombia’s legal and regulatory measures to increase access to affordable medicines, including… Continue Reading