In May 2023, the World Health Organization (WHO) published the global vaccine market report. Underpinning this technical report on vaccine market dynamics was a strong push by WHO’s Director-General, Tedros Adhanom Ghebreyesus, for increased transparency and equity; transparency is mentioned… Continue Reading →
Articles 2023, August 7. We Need Smart Intellectual Property Laws for Artificial Intelligence: “One-size-fits-all” regulation will sideline medical and research benefits promised by the advent of artificial intelligence. Article by James Love in Scientific American. New EU AI legislation Comments… Continue Reading →
Update: S.2333 passed Senate HELP today on a 17-3 bipartisan vote, with the delinkage study included. On Thursday, July 20, 2023, the Senate HELP Committee will consider a bill to reauthorize the Pandemic and All-Hazards Preparedness and Response Act (PAHPA).… Continue Reading →
On Monday, 17 July 2023, Chile, on behalf of a group of 62 WHO member states called for a more transparent, inclusive process for civil society engagement in the WHO negotiations of a pandemic treaty at the Intergovernmental Negotiating Body… Continue Reading →
On the fourteenth of June, the European parliament passed sweeping legislation on the regulation of artificial intelligence. The parliament’s position was backed with an overwhelming majority of 499 votes in favor and 28 against, with 93 abstentions. The legislation, which… Continue Reading →
KEI-NTIA-AI-Accountability-RFC KEI response to NTIA request for comments on AI Accountability Policy, expressing concerns over trade agreement provisions which limit the transparency of source code and algorithms RE: Notice by the National Telecommunications and Information Administration on 04/13/2023, Document Citation:… Continue Reading →
On May 31, 2023, the US Copyright Office hosted a listening session on artificial intelligence (AI) and copyright. Below are notes from KEI’s oral statement. Copyright Office Listening Session on AI and Copyright KEI Statement May 31, 2023 My name… Continue Reading →
Today, eight non-governmental organizations sent the Biden Administration a letter of support for the administrative appeal of the request for the Department of Health and Human Services (HHS) to use its rights in the patents on the prostate cancer drug… Continue Reading →
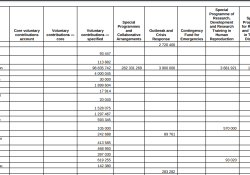
In advance of the 76th World Health Assembly (21–30 May 2023), the World Health Organization (WHO) published a document (A76/INF./5) on 24 April 2023 entitled, “Voluntary contributions by fund and by contributor, 2022.” Total voluntary contributions to WHO’s Total General… Continue Reading →
Media coverage of the WHO pandemic treaty negotiations (on transparency and content) – March/April 2023 2 February 2023, Reuters, Draft WHO pandemic deal pushes for equity to avoid COVID ‘failure’ repeat, By Jennifer Rigby and Gabrielle Tétrault-Farber. However, there will… Continue Reading →