March 27, 2020. We are writing to ask the WHO and its Member States to support the proposal by Costa Rica for the creation of a global pooling mechanism for rights in the data, knowledge and technologies useful in the… Continue Reading →
Below are Freedom of Information Act (FOIA) requests filed by KEI related to issues concerning the coronavirus/COVID-19 pandemic. This page will be updated as we file requests and receive responses. Department of Health and Human Services Centers for Disease… Continue Reading →
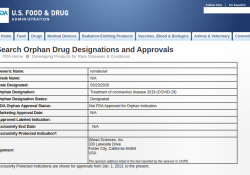
On Monday March 23, 2020, Gilead Sciences’ remdesivir received orphan designation from the US Food and Drug Administration (FDA) for the treatment of COVID-19. Remdesivir has been reported to be one of the candidates to potentially treat COVID-19, which the… Continue Reading →
A House coronavirus stimulus bill contains a provision concerning the use of Other Transactions Authority to fund COVID-19 diagnostics. The provision appears to authorize the Department of Homeland Security (DHS) to award $2.2 billion to private sector companies to develop… Continue Reading →
(KEI blogs and other work on COVID-19 are here: https://www.keionline.org/coronavirus) A letter from Costa Rica, signed by Carlos Alarado Quesada, the President, and Dr. Daniel Salas Peraza, the Minister of Health, to Dr. Tedros Adhanom Ghebreyesus, was sent this evening… Continue Reading →
Today the FDA granted Gilead Orphan Drug status for remdesivir for the treatment of COVID-19, on grounds this is a rare disease. The morning of the designation, the U.S. had confirmed, through testing, more than 35 thousand cases, including 8,477… Continue Reading →
For more on KEI’s work on COVID-19, see keionline.org/coronavirus. On March 23, 2020, Knowledge Ecology International (KEI) sent a letter to Speaker Nancy Pelosi regarding a provision in the current draft of the Senate version of the coronavirus funding bill… Continue Reading →
KEI Briefing Note 2020:1: Role of the U.S. Federal Government in the Development of GS-5734/Remdesivir Kathryn Ardizzone. March 20, 2020. (Updated May 28, 2020) KEI-Briefing-Note-2020_1GS-5734-Remdesivir
For more on KEI’s work on COVID-19, see keonline.org/coronavirus. Today, the Education, Culture, Science and Technology Commission of the National Assembly in Ecuador approved a resolution asking the Minister of Health to issue compulsory licenses over patents related to coronavirus… Continue Reading →
COVID-19 Gov’t Contracts: KEI has obtained numerous contracts between the federal government and pharmaceutical companies regarding COVID-19 medical technologies via the Freedom of Information act. For these and other contracts, please see: https://www.keionline.org/covid-contracts. COVID-19 Vaccine Manufacturing: KEI has gathered data… Continue Reading →