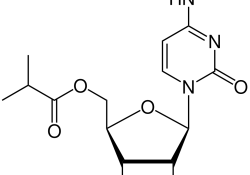
(For more resources, please see our page on molnupiravir.) The Medicines Patent Pool (MPP) and Merck Sharp & Dohme (“MSD” or “Merck”) have announced a voluntary license for the generic manufacture and sale of molnupiravir. A joint press release from… Continue Reading →
(For more resources, please see our page on molnupiravir.) According to Brook Baker, these are the countries included in the Merck voluntary license to generic suppliers Afghanistan, Algeria, Angola, Bangladesh, Belize, Benin, Bhutan, Bolivia, Botswana, Burkina Faso, Burundi, Cambodia, Cameroon,… Continue Reading →
(For more resources, please see our page on molnupiravir.) KEI has built a dataset of patents related to molnupiravir based upon a study by Imran et al (2021), the Medicines Patent Pool (MPP) MedsPal database, and our own searches. Our… Continue Reading →
Also known as EIDD-2801 or MK-4482. KEI Blogs 2021. October 22. Countries included in the Merck/molnupiravir license. 2021. October 20. International landscape of molnupiravir patents. 2021. October 4. U.S. Government’s $1.2 Billion Contract for Merck’s Investigational COVID-19 Drug Molnupiravir Redacts… Continue Reading →
(For more resources, please see our page on molnupiravir.) Molnupiravir, the oral pill that is showing promising results as a potential treatment for covid-19, was invented at Emory University with U.S. government funds. After more than six years of non-clinical… Continue Reading →
On March 18, 2015, KEI, KEI Europe, and Essential Inventions submitted proposals for global voluntary licences for all patents necessary for hepatitis C (HCV) medicines to five drug companies — AbbVie, Bristol-Myers Squibb, Gilead, Janssen, and Merck.
Continue Reading →
Monday, 30 April 2007 “The sanctioning of countries for using legitimate and important flexibilities in the TRIPS agreement brings shame to all U.S. citizens who are increasingly seen in Thailand and elsewhere as bullies and hypocrites.” The following is the… Continue Reading →
On Friday, March 16, KEI organized a briefing in the U.S. Capitol on Thailand’s recent compulsory licenses on three drugs; two for HIV/AIDS (Merck’s efavirenz (Stocrin) and Abbott’s lopinavir + ritonavir (Kaletra)) and one for heart disease (Sanofi’s clopidogrel (Plavix)). … Continue Reading →
Knowledge Ecology International: Q&A Session on Thai White Paper (Facts and Evidences on the 10 Burning Issues Related to the Government Use of Patents on Three Patented Essential Drugs in Thailand) Geneva, Switzerland 8 March 2007 Thiru Balasubramaniam On Thursday,… Continue Reading →
R 281104Z JUN 05 – June 28, 2005FM AMEMBASSY BRASILIATO SECSTATE WASHDC 1902SUBJECT: BRAZIL GIVES ABBOTT TEN DAYS BEFORE BREAKING PATENT 1. (U) SENSITIVE BUT UNCLASSIFIED 2. (U) Summary. On Friday June 24, the GoB announced a declaration of “public… Continue Reading →