On October 2, 2023, Knowledge Ecology International submitted written comments to the Centers for Medicare & Medicaid Services (CMS) in conjunction with the Medicare Price Negotiation public consultation process. CMS is hosting a series of patient-focused listening sessions this fall,… Continue Reading →
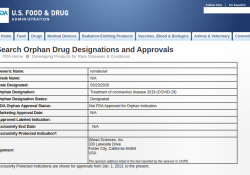
On Monday March 23, 2020, Gilead Sciences’ remdesivir received orphan designation from the US Food and Drug Administration (FDA) for the treatment of COVID-19. Remdesivir has been reported to be one of the candidates to potentially treat COVID-19, which the… Continue Reading →
Today the FDA granted Gilead Orphan Drug status for remdesivir for the treatment of COVID-19, on grounds this is a rare disease. The morning of the designation, the U.S. had confirmed, through testing, more than 35 thousand cases, including 8,477… Continue Reading →
Orphan Drug Timeline Data from Orphan Drug Tax Credit 2017. What does the Orphan Drug Tax Credit tell us about the Costs of Clinical Trials? Bill of Health. November 15. From 2010 to 2016, the average qualifying trial costs claimed… Continue Reading →
CPT has been highly critical of the exclusive marketing provisions in the US and the proposed EU orphan drug acts. Pharmaceutical companies now obtain 20 year patents for inventions, and investments in clinical trials and other research required for drug… Continue Reading →
(More on this topic here: https://www.keionline.org/orphan-drugs and here.) This is from: United States Senate, Committee on the Judiciary, Subcommittee on Antitrust, Monopolies and Business Rights, Anticompetitive Abuse of the Orphan Drug Act: Invitation to High Prices, January 21, 1992, Serial… Continue Reading →